Notes on Corrosion
Understanding Galvanic Corrosion
linkLast month, I began an overview of bonding and grounding issues by defining the terms used when dealing with the connection of conducting materials that are not part of the electrical utilization system. Once again: the bonding (the intentional interconnection of separate metallic components) and grounding (the intentional connection of a metal or system of metal components to a specific reference point) of the non-current carrying metal parts of a boat may accomplish three specific and separate objectives:
- Reduce the corrosive effects of dissimilar metal galvanic corrosion on expensive and critical boat parts.
- Eliminate electrolysis because of being the "ground" for another boat or an entire marina because of wiring defects.
- Protect persons and equipment from damage because of lightning.
When two different types of metal are in contact and subject to a corrosive environment, the least noble metal will be sacrificed. This is known as galvanic corrosion. (John Vigor's Practical Mariner's Book of Knowledge is an excellent source to identify the galvanic series of metals in seawater and most other essential sailing data.) A corrosive environment would include submersion in saltwater (and even freshwater), or the incidence of seawater spray.
The process of accelerated corrosion begins because of an exchange of ions, electrons and other atomic and subatomic particles at the point where these metals touch. This exchange of particles at the junction of these metals causes an electrical difference of potential between the metals. (This term is actually the root of all evils discussed in this article, and thus it needs to be fully understood.)
One way to understand "difference of potential" is to know that it can be measured in volts. In the case of the dissimilar metals that are in contact, the difference of potential is generated chemically. So what we are dealing with is a battery of sorts. Electrical current will flow if a connection or circuit is complete between the metals having a difference of potential.
A common lead-acid battery ceases having a difference of potential across its terminals, or is dead when the lead oxide plates are chemically converted to lead sulfate through use and discharge of the battery. A brass plumbing fitting threaded into a Monel bilge tank will cease to have a difference of potential with each other when all the zinc in the brass corrodes away and the remainder of the fitting crumples into dust.
In the case if submerged dissimilar metals, the more anodic (or less noble) metal corrodes and gives up part of its structure (in the form of metal ions conducted through the seawater) to the more cathodic (or more noble) metal. The anodic metal is essentially consumed. If you read my article last month ("Understanding Grounding and Bonding"), you'll recognize that this information repeats a portion of that article, but understanding this concept is extremely vital to solving both the induced voltage problems of galvanic action and the imposed voltage problems of both lightning and electrolysis. (We'll cover the latter two in a future article.)
Sacrificing the Cheap Stuff
Most sailors understand that the least noble anodic metal is sacrificed when a difference of potential is present between the electrically connected metals - that's what zincs are essentially all about. But fewer sailors know that the cathodic metal is actually protected from corrosion even though it is submerged in corrosive seawater. So, utilizing this principle, you can keep your boat from becoming a rustbucket in three steps:
- Use only very noble metals for critical parts and parts exposed to the most corrosive environment. Underwater parts should be made of silicone bronze or better. Stainless steel should not be embedded or otherwise sealed off from the atmosphere, as oxygen-starved stainless steel is much less noble than stainless steel that is exposed to air or water. And do not use common brass or mild steel for any permanent parts making up the structure of the vessel or its systems.
- Connect every metal part of a vessel to every other metal part of the vessel. This may seem like asking for trouble; after all, isn't one of the conditions of galvanic corrosion between dissimilar metals that they be in contact? How then can linking them together be a solution?
- Place sacrificial anode(s), connected to the bonded metal system, in contact with the seawater. These are usually made of zinc, a pretty non-noble metal and one that's relatively inexpensive.
Now, with everything important on board made of noble metal, and all of it connected together, the zinc anodes in the water are doomed! They will succumb to the very pox we are attacking - dissimilar metal corrosion. But the nature of this galvanic corrosion protects the more noble metals in the water that are connected to the sacrificial zinc. Once you realize how this works, you'll begin asking yourself, 'how many other problems in life end up having the solution already in place?'
Understanding How Metals Corrode Can Help Build Better Structures
linkExcept for the "precious" metals, such as gold, metals in the refined form are inherently unstable. This instability is what drives the process of corrosion, and it results from the fact that a refined metal is continually trying to revert to its natural state (the mineral). Some metals do this faster than others.
The Galvanic Series ranks corrosion tendencies in specific environments. The Galvanic Series for seawater is a much-used ranking because it's a good, general approximation of how metals behave. See Table 1.
How surface reactions alter a metal's corrosion resistance can be seen in the example of four common construction metals - aluminum, lead, copper and iron. Aluminum ranks as a very active, or corrosion-prone, element in both the AMF Series and the Galvanic Series for seawater, yet it is prized for its low maintenance and slow corrosion rate. This is because aluminum forms a tightly adhering surface film of aluminum oxide when exposed to the air. Under most atmospheric conditions, the oxide protects the aluminum from further corrosion. An exception is found in seashore locations.
When exposed to damp, salty air, most aluminum alloys behave very actively. Sea salt (mostly sodium chloride) destabilizes the normally protective oxide film, leading the localized attack, or "pitting." The reaction is so strong that a thin-gauge aluminum sheet will show perforation after being immersed in warm salty water for only a short period of exposure. However, not all aluminum alloys react so strongly to salt air. Aluminum masts, for example, are very popular on sailboats, but the alloy found in most aluminum flashing, roofing and siding does not stand up to salt, and should not be used near the sea. Aluminum performs much better in industrial atmospheres, although the top choices there are lead and copper.
Lead also forms a surface film of corrosion when exposed to the air. Because this film bonds so tightly with the underlying metal, however, it becomes a barrier to further corrosion. The types of films that form on lead include sulfate, oxides, and carbonates. Lead reacts with sulfur-bearing industrial atmospheres to produce lead sulfate, so it becomes very corrosion-resistant in industrial atmospheres and in areas subject to acid rain.
The green patina seen on older copper structures is a corrosion product consisting of copper sulfates and copper carbonates. The presence of sulfate films means that copper, like lead, holds up well in industrial atmospheres. But there is evidence that atmospheric corrosion of copper, while low, is increasing. Some old-timers remember that the green patina used to take about 25 years to form. It now forms in about 10 years, showing an increased corrosion rate in the underlying metal (although the green patina protects the underlying metal, it does not completely stop the corrosion). Observations of Christ Church in Philadelphia, for example show that its more than 200-year-old copper roof has an annual corrosion rate lower than that seen in contemporary structures.
While lead and copper serve well in heavily industrial atmospheres, zinc and galvanized steel fare poorly under the same conditions. Unlike aluminum, however, zinc and galvanized steel are the metals of choice in seacoast locations, where they suffer little damage from salt-heavy air.
Uncoated iron and steel are quite a different story. Although they are ranked midway in the EMF Series, indicating that they're mildly active metals, they are next to aluminum in the Galvanic Series for seawater. The active behavior of iron and steel results from the type of native oxide that they form. In contrast to the dense, tightly adhering films associated with aluminum, lead and copper, iron and steel oxides tend to be loose, porous, and nonadhering. The oxide flakes off almost as soon as it forms, exposing a fresh metal surface to further oxidation and attendant loss of metal.
An exception to this are products developed called "weathering steel" which modifies the oxide by alloying steel with copper to make the surface film more adherent, thus providing protection. Weathering steel will corrode, but it will usually do so more evenly and at a much slower pace than steel.
It cannot be overemphasized that the corrosion resistance of a metal depends on its naturally forming surface film, as well as on whether or not the film is protective. But corrosion is a complex subject, and several variables can influence a particular metal's performance. Local experience in different regions with each material is usually the best guide to its suitability for a particular use.
Galvanic corrosion
The Galvanic Series assume freely corroding metal, unaffected by contact with any other substance. Galvanic corrosion is a form of electrochemical corrosion that occurs when two dissimilar metals come together in the presence of an electrolyte to form an electrical couple, known as a galvanic couple. In building systems, the electrolyte is usually ordinary moisture, whether rainwater of high atmospheric humidity.
When two metals form an electrical couple, an exchange of electrons takes place, its direction and intensity governed by each metal's ranking in the Galvanic Series. The farther apart the two metals are on the Galvanic Series, the greater the potential for corrosion (see Table 2). This exchange protects the more noble (less active) metal, while causing the more active metal to corrode even faster. The more active metal gives up electrons, sacrificing itself to protect the more noble. We call the active, corroding metal the "anode" and the noble, non-corroding metal the "cathode". After the anode corrodes completely away, the cathode will again begin to corrode as reflected by its position in the Galvanic Series.
Although builders rightly see galvanic couples as something to be avoided, the process has its uses. Boaters, for example, use sacrificial anodes - buttons or bars of an aluminum magnesium alloy that corrode instead of more desirable metallic boat parts - to protect engine parts or propellers. And galvanic couples are the mechanism by which galvanizing works.
Galvanizing means simply overlaying steel with zinc, either by plating or by dipping the steel in molten zinc. An undamaged piece of galvanized steel will corrode at the same rate as a similar piece of zinc. Once the zinc coating is perforated (by mechanical damage, for example), the zinc forms a galvanic couple with the steel, the zinc corroding to protect the steel. The zinc will continue to protect the steel until most of the zinc is gone.
When the zinc is gone, you may begin to see a lot of thin patches of rust. What this means depends on whether the zinc was applied by plating or dipping. On an electroplated surface, such as a galvanized-metal roof, the rust indicates that corrosion of the underlying metal has begun. On a hot-dipped galvanized surface, however, the zinc actually diffuses partway into the steel. The initial patches of rust mean that the pure zinc overlay has corroded away. Thus a piece of hot-dipped galvanized steel will give you some warning before the steel begins to corrode.
By Ana Diaz
The Galvanic Series for Seawater
| Most cathodic, or passive |
|
| Most anodic, or active |
|
Galvanic corrosion potential between common construction metals
| Alum. | Brass | Bronze | Copper | Galvan Steel | Iron/ steel | Lead | Stain steel | Zinc | |
|---|---|---|---|---|---|---|---|---|---|
| Aluminum | 1 | 1 | 1 | 3 | 2 | 2 | 3 | 3 | |
| Copper | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | |
| Galvanized steel | 3 | 2 | 2 | 2 | 2 | 3 | 3 | ||
| Lead | 2 | 2 | 2 | 2 | 3 | 3 | 2 | 3 | |
| Stainless steel* | 3 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | |
| Zinc | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | |
|
|||||||||
Metal Corrosion Basics
linkThis article explains the metal corrosion process including the basic chemistry of how metal loss occurs. Methods to control corrosion are also listed and explained.
The Corrosion Process
Metal corrosion is a chemical reaction between a metal surface and its environment. Corrosion can occur in a gaseous (dry) environment or a damp (wet) environment. Figure 1 shows the behaviour at the atomic level of both dry and wet environmental corrosion.
Corrosion in a gaseous environment produces a surface layer of converted metal. For example atmospheric corrosion of zinc produces the dull, gray zinc oxide layer seen on galvanised street light poles. Unoxidised zinc coating fresh from the hot dip galvanisers is bright and shiny.
Corrosion in a wet environment attacks the metal by removing the atoms on the metal surface. The metal atoms at the surface lose electrons and become actively charged ions that leave the metal and enter the ‘wet’ electrolyte. The metal ions join with/to oppositely charged ions from another chemical and form a new, stable compound.
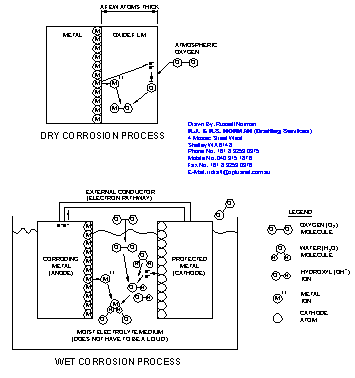
Corrosion requires energy. During corrosion the reacting components go from a higher to a lower energy state and release the energy needed for the reaction. In the dry corrosion process of Figure 1 the metal and the oxygen combine to produce the oxide on the surface because the reaction leads to a compound (the oxide) at a lower energy level.
The oxide layer shields the metal from the oxygen and forms a barrier. The oxide will not react with the oxygen in the air or the metal. The barrier makes it difficult for oxygen in the air to contact the metal and it eventually grows so thick that the movement of electrons and ions across it stop. Provided the oxide layer does not crack, or is not removed, the metal is protected from further corrosion. But if the bare metal is exposed to the oxygen, it will again react to form the oxide. In this case the presence of oxygen benefits the metal's protection. Removal of the oxygen removes the metal's ability to create its own protective corrosion barrier.
In the wet corrosion process of Figure 1 the electrons from the corroding anode metal move to the connected cathode where they recombine with the atoms of oxygen and water in the electrolyte to make a new hydroxyl ion (OH-). This new negatively charged ion then reacts to make a stable compound with the positively charged metal ions (M++) that originally lost the electrons. In this case, the electrons have a continuous pathway to escape the parent metal and the parent metal, which cannot develop a protective barrier, disassociates or falls apart. Once corrosion starts it continues until the ingredients are all used up.
The electrolyte in wet corrosion can be neutral, acidic or alkaline. For corrosion in near neutral solutions (pH 6 – 8) under oxygenated conditions the predominant cathodic reaction is the oxygen absorption reaction (O2 + 2H2O + 4e- = 4OH-) shown in Figure 1. If instead the bimetallic cell has no oxygen present in the electrolyte the hydrogen evolution reaction (H+ + e- = H followed by H + H = H2 gas) becomes the cathodic process and the anode continues to corrode. This reaction is a much slower reaction (the H+ ion has a very low concentration in solution) than the oxygen absorbing reaction. In acidic solutions (pH 0 - 6) the hydrogen ion concentration is higher and the hydrogen evolution reaction is the predominant one. Corrosion rates become extreme as the pH drops (acid gets stronger).
The Electrical Nature of Corrosion
A flow of electrons means there is an electric current. Wet corrosion produces a corrosion cell. Much like a car battery. The electrons used in creating the corrosion product are continually replaced from the corroding metal. The numbers of electrons available for reacting control the amount of current developed between the two metals. The anode cannot corrode unless there is a cathode. One of them will control the rate of electron flow and thus the corrosion rate.
The intensity (the number of electron and positive ion pairs) is dependent on the potential difference, or voltage, which exists between the metals and the surface area of each metal. Different metal combinations have different voltage potentials between them. Joining two metals with a large potential difference between them produces higher corrosion rates than if the metals were close in electrical potential.
Surface Area Effects
The size of the cathode relative to the anode is important. A large cathode has more surface area through which electrons can flow and so develops an intense electric current with the anode (corroding metal). A small anode connected to it is forced to supply these electrons and will quickly corrode and fall apart. Whereas a large anode connected to a small cathode can provide electrons from any location and will take a long time to show evidence of corrosion.
Where a less noble (base, anodic) metal has to be in contact with a noble metal make sure the less noble metal has at least one hundred times more surface area than the noble metal. Remember – large anode, small cathode – not the opposite.
Differential Aeration Effects
The corrosion reaction requires oxygen and where oxygen is present the metal is cathodic and where oxygen is depleted the metal is anodic and corrodes. The parts of the metal in contact with the highest oxygen concentration become cathodic and are protected, and the areas where oxygen concentration is low will corrode. Steel posts dug into the ground will rust just below the surface because of this effect.
Stagnation Effects
During corrosion, ions build up immediately around the anode and cathode saturating their respective regions. The corrosion rate begins to fall due to the concentration of stagnant ions blocking the creation of more ions in the electrolyte. If the ions are removed or more voltage is provided the corrosion rate again picks up. If you want fast corrosion then agitate the electrolyte and add oxygen.
Specific Types of Corrosion
Corrosion produces physical evidence of its presence. The form it takes depends on the mechanism of the corrosion. Some of the more common forms are explained below.
Pitting Corrosion
A metal can corrode without being in contact with another metal. In this case different areas of the metal take on different electrical potentials. This can occur because of variations in the metal metallurgical properties or because of variations in the surface oxide layer, such as a break, thinning, inclusion like mill scale, contaminant like dirt, etc.
In pitting corrosion the metal at the top of the pit has access to the oxygen in the air and becomes the cathode. At the bottom of the pit oxygen is depleted and the metal becomes the anode. The deeper the pit is the less the oxygen available at the bottom and the corrosion rate increases. Figure 2 shows the mechanism of pitting corrosion.
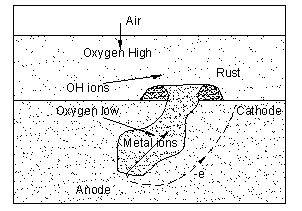
Crevice Corrosion
A crevice is created whenever two objects are brought together. Unless they are perfectly flat a crevice is present and oxygen cannot easily enter the gap but is plentiful outside. Corrosion starts in the crevice because of differential aeration. Figure 3 is a drawing of crevice corrosion occurring under a layer of seawater.
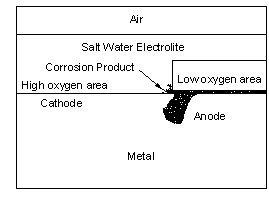
Stress Corrosion
Metal under tensile stresses can corrode at higher rates than normally expected. The stressed areas have changed electrical potentials to the neighbouring metal and are also more likely to develop microscopic surface cracks. Both situations promote increased corrosion rates.
Bacterial Corrosion
There exist many species of bacteria living in moist environments that release acidic waste products or that can strip out elemental components of a metal. If these bacteria grow on pipe walls and metal surfaces they will cause corrosion. They occur in both oxygenated (aerobic) and oxygen free (anaerobic) conditions.
Galvanic Corrosion
Galvanic corrosion needs to be watched. Dissimilar metals of different potentials joined together by an electrolyte, like process water or rainwater, will cause the more anodic metal to corrode. Running copper water pipe to a galvanised tank will cause the tank to corrode very quickly. Joining copper to steel is nearly as bad. In the galvanic series listed in Table 1 only join metals that are near each other.
Some protection from galvanic corrosion can be achieved if the electrolyte is not present. Without the availability of water molecules the corrosion reaction stops because the electrons cannot find a host to complete the chemical reaction. Where dissimilar metals must be used, for example aluminum fins on the copper coils of a refrigeration chiller condenser, protect them from contact with water. If water must be used in contact with dissimilar metals insure it is deionised and oxygen free.
| Galvanic Series of Metals and Alloys in sea water |
Graphite |
|
Controlling Corrosion
Corrosion control involves hindering the natural chemical reactions that occur between the metal and its environment. The common methods used are to:
- Modify the environment
- Modify the properties of a metal
- Install a protective coating over the metals
- Impose an electric current to supply electrons
- Change to non-metallic materials
Modify the Environment
Removing oxygen from the environment prevents completion of the corrosion process by slowing the chemical reaction requiring electrons. If oxygen can be kept away from the protected cathode then the electrons cannot readily flow, so causing the current to drop and corrosion to slow.
Another technique is to use corrosion inhibitors that combine with the corroding metal (anode) or the protected metal (cathode) to form a barrier layer that reduces the flow of ions and electrons across it to very low values and virtually stops the corrosion. If the protective barrier layer is damaged corrosion restarts, so it is necessary to keep an amount of the inhibitor in contact with the metals. This is a common technique used in boilers to protect them from corrosion.
Modify the Properties of the Metal
From the galvanic series we can see that the more noble metals are less likely to corrode. When these metals are metallurgically combined with those from lower in the series, the resulting alloy takes on corrosion resistant properties. The resistance can come from the development of a protective oxide film on the outside surface or because the new alloy has a different voltage potential which acts to make it behave more noble.
Passivation of a metal is a method of changing the potential difference of a metal's surface. By removing the oxide layer normally present on a metal and exposing the bare metal directly with an acid, the acid reacts with the metal surface to make a new compound with more noble electrical properties. The passivated layer covers the metal, and provided the layer is not broken and the voltage potential remains favourable, it will protect the metal under it from corrosion.
Put Protective Coating Over the Metal
Metals can be protected by covering them in a coating of a different material with better corrosion resistant properties. Metallic and non-metallic coatings are used.
Metallic coatings of less noble metal over more noble metal provide sacrificial protection. Galvanizing is a bonded, protective coat of zinc put over steel. The zinc protects the steel from corrosion in two ways. From the galvanic series it can be seen that zinc will corrode before steel (sacrificial). Secondly, a protective layer of zinc oxide forms on the zinc. If the oxide layer is scratched the zinc is exposed to oxygen and the oxide layer reforms. If part of the zinc coat is lost the rest of the bonded zinc starts to corrode in preference to the steel. As long as the zinc remains in contact with the steel it corrodes sacrificially and protects the steel.
Non-metallic coatings put over a metal can be of two types. They can act as a physical barrier and bar access to the metal surface or they can introduce a very high resistance into the corrosion cell circuit and drastically reduce the flow of electrons. The barrier type coatings protect the metal as long as there are no cracks. If a crack occurs corrosion becomes intense at the metal surface. Resistance type coatings include additives that breakdown in the presence of water and oxygen into inhibiting agents.
Impose an Electric Current
Wet corrosion produces a bi-metallic cell and an electrical current of moving electrons flow from the less noble, anodic metal. If instead the electrons were supplied from another source the less noble metal would not corrode first. This technique is known as cathodic protection. By connecting a more anodic metal into the corrosion circuit than the metal to be protected, the more anodic metal will corrode first and provide an alternate source of electrons. This is why zinc blocks are placed on ship hulls to protect any steel in contact with seawater.
Alternatively a metered electrical current from a power source can be connected to the cathode to supply the electrons. Figure 4 shows cathodic protection installed on a below ground pipeline using sacrificial anodes that require regular replacement as they are corroded away.
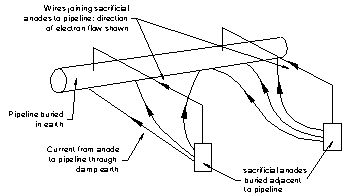
Change to Non-Metallic Materials
There are many other materials that can be used instead of metals in situations where metallic corrosion is expected. Provided the physical properties of the non-metal are satisfactory their use may prove a more effective choice.
Practices to Adopt to Reduce Corrosion
- Clean out debris from the bottom of metal sumps exposed to the atmosphere to prevent oxygen depletion under the debris.
- Repair any damage to painted surfaces quickly to seal the metal off from atmospheric oxygen and moisture.
- Don't create galvanic cells by mixing metals. Check the galvanic series when ordering equipment and parts to see if components are compatible. Keep them dry and out of the weather.
- Check the right type of paint is being used with good corrosion protective properties.
- Grout under base plates completely and finish the grout at the edges of the lower face. Do not bring it up the side of the base plate and create a crevice.
- Remove a paint blister immediately, clean the metal thoroughly and repaint to prevent differential aeration.
- Before bolting or clamping metals parts together insure they both have a completely protected surface. This can be with a sacrificial coating or a barrier coating.
- Be aware that electric currents can flow for hundreds of meters if parts are joined by an electrolyte. Underground pipes in moist soils need to be protected from bother oxygen depletion and galvanic action from dissimilar metals anywhere along their route.
- Use a generous radius when bending metals to minimise the creation of internal tensile stresses.
- Use deionised water when washing down components made of dissimilar metals in contact and dry them off quickly and thoroughly. This is especially important with coils made of dissimilar metals.
Protect underground piping from direct contact with soil and protect
the coating from damage. The smallest penetration to the metal will
result in rapid pitting corrosion of the pipe wall. If that is not
possible install cathodic protection. Beware that some painted coatings
still allow moisture and oxygen through to the metal. Check the
corrosion protection properties of the coating.
Mike Sondalini is the Maintenance Manager in one of Australia's most progressive medium sized mining and agricultural chemical manufacturing companies. He graduated with first class Honours in mechanical engineering from Curtin University and has been working as a professional engineer since 1985. He has since completed an MBA at the University of Western Australia. His experience includes 8 years with one of Australia's largest breweries in the positions of Project Engineer and later Brewing Engineer in charge of brewing, utilities and services maintenance. His current position puts him in charge of maintenance at sulphuric acid storage terminals, petroleum products storage terminals, chemical processing and bulk materials handling plants throughout Australia. He writes for Feed Forward's UP-TIME from a strong position of having substantial practical experience working together with his people in solving the 'everyday' maintenance problems in their plants.
An interesting homepage on stainless steels, all kinds of information.